Join the Interacoustics community and receive news about new products, events and much more
An introduction to Saccadometry
Table of contents
- What is Saccadometry?
- Methodology
- Parameters
- Clinical significance
- Mapping the world with saccades
- Using saccades to localize dysfunction
What is Saccadometry?
Saccadometry is an advanced ocular motor test that allows for the functional evaluation of the varied brain regions and circuits involved in the generation of fast, appropriate, purposeful, and accurate saccadic eye movements.
Saccades are traditionally analyzed using three different characteristics including, latency, amplitude, and velocity.
Saccadometry adds the analysis of phase data, which provides further insight for lesion localization, and antisaccade analysis that offers windows into cognition, emotional regulation, response inhibition, and executive function (1).
This deeper dive into the saccadic and antisaccadic systems can provide clinically actionable insights into neurological disorders such as concussion and traumatic brain injury, neurodegenerative diseases, movement disorders, depression, and attention-deficit disorders, among many others.
These more advanced tests provide information that is not always detected in the traditional saccade test.
Saccadometry methodology
The protocols are performed using the VisualEyes VNG system. The ocular motor stimuli are projected onto a large TV screen. The patient is seated in front of the screen. The background of the screen is black because high contrast screens are difficult for these patients to tolerate for the duration of the test.
The protocol settings are:
- Duration: 151 secs
- Number of jumps: 60
- Jump size: 10 degrees
- Mean time interval: 1.5 sec
- Number of dots: 2
- Artifact rejection: ON
- Jump pattern: RANDOMIZED
- Compensate for drift: ON
- Target colors: RED, RED, RED
- Background color: BLACK
- Enable practice mode: OFF
The subject is instructed to keep their head still and follow the targets with their eyes only.
For prosaccades the subjects are instructed to look to the center and then for each saccade jump they are to look at the new target and then return to the center dot and wait for the next jump.
For the antisaccades the subjects are instructed to look in the opposite direction of the saccade jump and then return to center and wait for the next jump.
The graphs below show the subject’s eye movements relative to the target movements.
The target is in yellow and the subject’s are in red for the right eye and blue for the left eye.
In the prosaccades you can see the subject looks towards the target jumps and in the antisaccade they are looking away from the target jumps.

Figure 1(a): Prosaccade.

Figure 1(b): Antisaccade.
Saccade parameters
During the test we are assessing the saccade parameters of latency, velocity and accuracy as in the standard random saccade test.
When the test is complete, we can see summary graphs for position, velocity, latency, phase and error rate.
There are two measurements under error rate:
- Directional error rate
- Overall error rate
Directional error rate is the percentage of times the patient moves their eyes in the incorrect direction, and overall error rate is a pulse on overall noise and artifact present in the recording.

Figure 2: Summary graphs in a left acoustic neuroma patient.
The green lines represent targets moving to the right, controlled by the left brain and the red lines represent targets moving to the left, controlled by the right brain.
The dotted lines represent jumps where the subject looked in the incorrect direction, hence causing an error to be recorded.
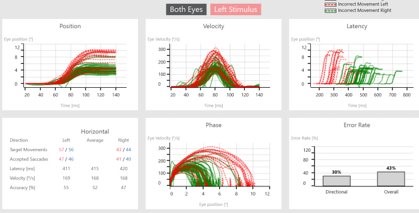
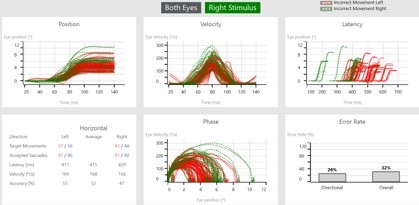
Figures 3 (a) and (b): Data shown by stimulus direction.
After a completed test, you can also visualize the data by stimulus direction. This can aid in a more detailed diagnosis and help to distinguish the side of lesion. The data shown in Figures 3 (a) and (b) is from a left acoustic neuroma patient.
Clinical significance of anti- and prosaccades
The ability to suppress reflexive responses in favor of voluntary motor acts is crucial for everyday life. The antisaccade test can assess both abilities; to suppress and to make the voluntary jump. This task requires subjects to suppress a reflexive prosaccade and instead to generate a voluntary saccade to the opposite side. Current data show that a variety of brain lesions, neurological diseases and psychiatric disorders result in errors, i.e. prosaccades towards the stimulus, in this task. Brain imaging studies have shown that a widely distributed cortical and subcortical network is active during the generation of antisaccades. Current data show that a variety of brain lesions, neurological diseases and psychiatric disorders result in prosaccadic directional errors (3-12).
Mapping the world with saccades
One of the primary tasks for our brain is to localize ourselves within the environment, which is a necessary condition to being able to respond to the world appropriately.
This requires a complex interplay between proprioceptive systems, which inform the brain of the status of stresses on muscles and joints, vestibular systems that inform the brain about the head’s position relative to gravity and the direction and velocity of head movement, and visual systems, which inform the brain of the body’s position and movement relative to the visual surround.
There are a host of visual reflexes involved in this process, but localization crucially relies on the ability to rapidly and accurately map the world with saccades.
Using saccades to localize dysfunction
Saccades utilize pathways involving the occipital lobe for vision, the parietal lobe for localization, and the frontal lobe for initiation.
Pathways descend from the frontal lobe through the basal ganglia to the brainstem superior collicular saccadic pulse generators that activate oculomotor systems to move the eyes to targets.
Inputs to these systems from several parts of the cerebellum modulate accuracy of saccades.
There are well-understood patterns of saccade impairment that allow clinical localization of dysfunction and lesions within these systems.
1. Antisaccades
Antisaccades utilize similar output circuitry as saccades, with the addition of modulation from tertiary prefrontal cortex systems that introduce a higher level of executive function to the process.
Key to the antisaccade task is the process of response inhibition, wherein the subject must suppress the reflexive prosaccade to the target, which is a function of the dorsolateral prefrontal cortex (DLPFC).
The DLPFC must then communicate with the opposite frontal lobe via the corpus callosum for generation of a saccade to a remembered target in the opposite direction.
Impairment of antisaccade function has been demonstrated in concussion and mTBI (3).
Failure of accurate antisaccades to memorized targets can demonstrate disruption of visual memory circuits that are crucially involved in working memory processes. Failure of response inhibition can reveal impairment of attentional control and executive function. The failed response inhibition demonstrated on antisaccade testing has also been demonstrated in ADHD (4).
The DLPFC has been demonstrated to be a critical region for the regulation of affect. Impaired antisaccade performance has been studied as a sensitive biomarker for psychological conditions ranging from chronic depression to schizophrenia (9, 6).
2. Abnormal latencies and velocities
The distributed nature of pathways and structures involved in prosaccade and antisaccade generation makes them very sensitive to damage in concussion and traumatic brain injury (3).
Increased latencies and expanded latency distributions have been shown to be sensitive signs of unresolved mTBI. Aberrancies in saccade velocities can imply dysfunction in brainstem generators. Lack of accuracy and changes in velocity can indicate damage to cerebellar systems, with overshoot vs undershoot aiding localization within discrete cerebellar systems (10).
3. Movement disorders
Aberrant patterns of saccade generation are well-represented in movement disorder literature. Changes in saccade phase can help localize lesions within the frontal-basal ganglia network. Changes in accuracy can reveal aberrancies in the superior collicular saccade maps that are crucial to appropriate motor output in movement disorders such as Dystonia (11) and Parkinson’s disease (12).
These parameters, when combined with directional changes in saccade velocity, can aid in appropriate diagnosis of Parkinsonian syndromes such as Progressive Supranuclear Palsy and Multiple System Atrophy (5).
4. Executive function disorders
Relationships of prosaccades and antisaccades can also provide insight into executive function disorders ranging from Mild Cognitive Impairment to Alzheimer’s disease and Frontotemporal Dementia (7, 8).
Summary
The addition of prosaccade and antisaccade protocols to our testing provides more than additional tools for lesion localization.
They allow us to precisely guide interventions for neurorehabilitation and assess fatigability of involved systems.
This facilitates superior functional outcomes from our therapies (2).
About the authors
Dr. Glen Zielinski is a Chiropractic Physician and Board-Certified Functional Neurologist. He received his doctorate in chiropractic from Parker University in Dallas, Texas. He completed his neurology training with the Carrick Institute for Clinical Neuroscience, and became board certified as a Chiropractic Neurologist by the American Chiropractic Neurology Board in 2003. He was appointed as an Assistant Professor of Neurology with the Carrick Institute later that year. He was awarded a fellowship from the American College of Functional Neurology in 2010. He is fellowship-trained in Traumatic Brain Injury Rehabilitation, Vestibular Rehabilitation, Movement Disorder Rehabilitation, Childhood Neurodevelopmental Disorder Rehabilitation, and Clinical Neurochemistry. Dr. Zielinski has lectured for thousands of hours on Functional Neurology to chiropractic, medical, osteopathic, and naturopathic physicians throughout North America and Europe. He has qualified hundreds of doctors throughout the world to sit for board examination in Functional Neurology. Dr. Zielinski founded Northwest Functional Neurology in Lake Oswego, Oregon, in 2006. His practice focuses on severe traumatic brain injuries, post-concussion syndrome, movement disorders, disorders of gait and balance, and disorders of childhood neurological development.
Michelle Petrak, Ph.D., is the Director of Clinical Audiology and Vestibular Research for Interacoustics. Her primary role is development and clinical validation of new technologies in the vestibular and balance areas. She is located in Chicago where she is a licensed private practice clinical audiologist at Northwest Speech and Hearing (NWSPH). Dr. Petrak received her doctorates in Electrophysiology (1992) and Biomolecular Electronics (1994) from Wayne State University and her Masters in Audiology in 1989. Her special areas of expertise include vestibular and balance assessments and management of the dizzy patient. Dr. Petrak is involved with new innovative product developments, clinical evaluations of new protocols, and publishing, teaching and training on the management of patients with dizziness. She continues to lecture extensively nationally and internationally, and she has numerous articles published in the hearing industry journals. She also participates on the committees for several doctoral students as support for the research projects.
Dr. Liz Fuemmeler, Au.D., is a Clinical Product Manager with Interacoustics and Vestibular Program Director at Professional Hearing Center in Kansas City, MO.
References
1) Munos D, Everling S. Look Away: The Anti-Saccade Task and Voluntary Control of Eye Movement. Nature Review, Neuroscience. 2004, 5:218-228.
2) Carrick F, Hankir R, Saman R, Antonucci M, Pagnacco G, Azzolino S and Oggero E: Improvement of Saccadic Eye Movements After Head-Eye Vestibular Motion (HEVM) Therapy and Neuro-Psychiatric Considerations. Psychiatry Danub. 2019 Sep;31(Suppl 3):318-323.
3) Kwan-Chun Ting W , Schweizer T , Topolovec-Vranic J and Cusimano M: Antisaccadic Eye Movements Are correlated with Corpus Callosum White Matter Mean Diffusivity, Stroop Performance, and Symptom Burden in Mild Traumatic Brain Injury and Concussion. Frontiers in Neurology, 2016, 6:271.
4) Munoz D, Armstrong I, Hampton K and Moore K: Altered Control of Visual Fixation and Saccadic Eye Movements in Attention-Deficit Hyperactivity Disorder. J Neurophysiol 90: 503–514, 2003.
5) Termsarasab P, Thammongkolchai T, Rucker J and. Frucht S: The diagnostic value of saccades in movement disorder patients: a practical guide and review. Journal of Clinical Movement Disorders (2015) 2:14.
6) Rodrigue A, Austin B, Dyckman K and McDowell J: Brain activation differences in schizophrenia during context‑dependent processing of saccade tasks. Behavioral and Brain Functions, (2016) 12:19.
7) Heuer H, Mirsky B, Kong E, Dickerson B, Miller B, Kramer J and Boxer A: Antisaccade task reflects cortical involvement in mild cognitive impairment. Neurology 81 October 1, 2013.
8) Yang Q, Wang T, Su N, Xiao S and Kapoula Z: Specific saccade deficits in patients with Alzheimer’s disease at mild to moderate stage and in patients with amnestic mild cognitive impairment. AGE (2013) 35:1287–1298.
9) Hoffmann A, Ettinger U, Montoro C, Reyes Del Paso G and Duschek S: Cerebral Blood Flow Responses During Prosaccade and Antisaccade Preparation in Major Depression. European Archives of Psychiatry and Clinical Neuroscience, 2019 Oct;269(7):813-822.
10) Jensen K, Balta Beylergil S and Shaikh A: Slow saccades in cerebellar disease. Cerebellum & Ataxias (2019) 6:1.
11) Beck R, Kneafsey S, Narasimham S, O'Riordan S, Isa T, Hutchinson M and Reilly R: Reduced Frequency of Ipsilateral Express Saccades in Cervical Dystonia: Probing the Nigro-Tectal Pathway, 2018 Nov 16;8:592.
12) Shaikh A, Ghasia F: Saccades in Parkinson's Disease: Hypometric, Slow, and Maladaptive. Progress in Brain Research, 2019;249:81-94.
Similar Topic
Stay up to date!
Subscribe to our newsletter and receive news on new products, seminars and much more.
By signing up, I accept to receive newsletter e-mails from Interacoustics. I can withdraw my consent at any time by using the ‘unsubscribe’-function included in each e-mail.
Click here and read our privacy notice, if you want to know more about how we treat and protect your personal data.
