Subscribe to the Interacoustics Academy newsletter for updates and priority access to online events
Training in OAE
Distortion Product Otoacoustic Emissions (DPOAE) Interpretation Protocol for Infants and Children
Description
Lisa L. Hunter, PhD1, Chelsea M. Blankenship1, AuD, PhD and Yvonne S. Sininger2, PhD
1Cincinnati Children’s Hospital Medical Center, Division of Patient Service Research, Children’s Audiology Research Laboratory, Cincinnati, Ohio, USA 45229
2C & Y Consulting LLC, Sante Fe, NM USA 87506
Contact author:
Introduction
When audiologists assess infants and children for hearing loss, otoacoustic emissions are a vital tool in combination with auditory brainstem response (ABR) or behavioral evaluation once reliable responses can be obtained. As with any test, it is crucial to have clinically validated, age-appropriate normative ranges to use for diagnostic purposes. The most-often used clinical diagnostic protocol for evaluating distortion product otoacoustic emissions (DPOAEs) is based on studies in populations of primarily adults (Gorga et al., 1997; 1999; Dorn et al., 1999; Gorga et al., 2005). These landmark studies validated behavioral audiometry as the clinical standard for DPOAE to predict presence of hearing loss in large samples across a wide age range (1 year to 96 years). They also introduced a DPOAE interpretation approach using signal-to-noise ratio (SNR) to determine that a response was present and above the noise floor, combined with DPOAE level below the normal range for detection of hearing loss. Multivariate formulas weighted for different frequencies have been recommended for optimal interpretation of DPOAEs (Dorn et al., 1999; Gorga et al., 2005).
How should DPOAEs be interpreted to predict hearing loss in infants and children?
Most clinical audiologists and many studies use a set criterion across frequencies of SNR varying from 3 to 8 dB, with 6 dB as the most common criterion for presence of DPOAE interpretation, regardless of age. Most clinics interpret SNR >6 dB at the majority of frequencies tested as indicative of “no more than a mild hearing loss”. However, it is important to know that SNR is best used to determine the presence of a valid OAE, not to assess risk for hearing loss, as DPOAE level is more sensitive and specific for assessing risk for hearing loss.
As instrumentation has improved over the years for averaging methods to decrease noise, relying on SNR alone is insufficient in infants and children, who have larger DPOAE levels than adults. Due to this age effect, in a quiet infant SNR can exceed 6-8 dB despite abnormally low (for age) OAE levels. Further, SNR is more variable across different makes and models of instruments due to varying algorithms for noise reduction, while DPOAE level is more consistent across instruments because it is primarily dependent on hair cell function, rather than body or environmental noise. This means that detection of mild hearing loss in infants may be inadequate using SNR alone.
One study of 118 premature infants who passed newborn hearing screening and were evaluated at various ages reported significant differences between infant and adult DPOAE levels and noise floors (Abdala et al., 2008). DPOAE levels were larger in 6-month-old infants compared to adults, ranging from 6 dB greater at 1500 Hz to 14 dB greater at 8 kHz. DPOAE levels were also slightly larger in the infants at 6 months compared to term birth. This study was important for documenting normal DPOAEs in infants, although comparison with defined hearing loss was not available, and it was unclear whether results were equivalent in full term infants.
Are normative studies available in infants and children?
Until recently, published studies have not been available in infants and young children that provided normative ranges of diagnostic DPOAE interpretation with validation of hearing levels. We have sought to fill that gap with studies that were supported with grants from the National Institutes of Health and from the William Demant Hearing Foundation. The results are described below. Another limitation of previous studies of DPOAE in young infants is they did not include a test of middle ear function, or used 226-Hz tympanometry, which is not sensitive to middle ear effusion in young infants. A gap in knowledge from the studies discussed thus far, and an important question to address is “What are appropriate diagnostic DPOAE criteria to use for infants and young children to detect all degrees of hearing loss, including slight-mild?” To address this question, we have completed three studies in newborns through age 6 years with hearing status validated using threshold ABR or age-appropriate behavioral hearing assessment coupled with wideband tympanometry measures (Hunter et al., 2018; Blankenship et al., 2018; Hunter and Sininger, 2021).
Study 1:
Study 1 was an NIH-funded, prospective longitudinal study (Hunter et al., 2018) of 231 infants who passed newborn hearing screening and were verified to have normal hearing with threshold toneburst ABR and later with visual reinforcement audiometry, while middle ear function was measured using wideband tympanometry. Both full-term and premature infants were included. DPOAEs were assessed longitudinally in four study visits over the first 15 months after birth at six f2 frequencies presented in order from high to lower frequencies (8, 5.5, 4, 3, 2, 1.5, and 1 kHz). Primary tone stimulus levels were set at SPLs of 65 dB (L1) and 55 dB (L2) with a primary tone f2/f1 frequency ratio of 1.22. Average DPOAE levels for this study were consistent with results reported by Abdala et al (2008) but were much higher than in adults (Gorga et al., 1996). Other findings from this study were that DPOAE levels were highest at 1 month of age, then decreased slightly between 1 and 5 months of age in the mid to high frequencies (2 to 8 kHz) with minimal additional changes occurring between 6, 9, and 12 months of age. The decrease in DPOAE level was related to a decrease in wideband absorbance at the same f2 frequencies, suggesting that development of middle ear function affected DPOAE levels in infants. DPOAE noise level increased only slightly with age over the first year with the highest noise levels in the 12-month-old age range. Only minor effects for NICU history, race, and gestational age at birth were found, thus these results were generalizable to commonly seen clinical populations.
Study 2:
Study 2 analyzed the normal hearing infants from study 1, plus infants with hearing loss to determine the best criteria for detection of mild or greater hearing loss. In total, 279 infants with and without defined hearing loss were included (Blankenship et al., 2018). Infants received a complete audiologic assessment at about 1 month of age with DPOAEs (1–8 kHz), wideband absorbance tympanometry (0.25–8 kHz), and air and bone conduction diagnostic tone burst auditory brainstem response (0.5–4 kHz) thresholds. Hearing status was verified at 9-12 months of age with visual reinforcement audiometry (0.5–4 kHz). Auditory brainstem response air conduction thresholds were used to assign infants to a normal hearing (NH) or hearing loss (HL) group, and efficacy of DPOAE results to classify ears as NH or HL (conductive, sensorineural, or mixed losses) was assessed, balancing optimal detection of hearing loss and minimizing false-positive results.
Predictive accuracy of DPOAEs was best at mid to high frequencies (3–8 kHz) with intermediate performance at 1.5 and 2 kHz and chance performance at 1 kHz. Infants with a conductive component had significantly lower ambient wideband absorbance values than the NH group. No differences in ambient wideband absorbance were found between the NH and SNHL groups. Multifrequency analysis resulted in the best prediction of HL. Interpretation of DPOAEs for hearing loss type was achieved when combined with wideband tympanometry. Importantly, in this large infant study, compared to normative ranges in common clinical use (Gorga et al., 1997, DPOAE levels ≥ -10 dB on average), more than 90% of the frequency-specific results in cases with hearing loss would have been classified in the normal or borderline ranges. Testing frequencies below 2 kHz was not recommended due to poorer predictive value and longer test times necessary to achieve adequate signal to noise ratios. For optimal classification of hearing loss, clinical criteria were recommended.
Study 3:
Study 3 was a multisite clinical study funded by the Oticon Hearing Foundation. It included infants and children up to age 7 years using the Interacoustics Titan DPOAE system (Sininger et al., 2018). In this study, 85 children (age 0.7 to 80 months; mean=12.6 mo.) were evaluated using CE-Chirp narrowband ABR thresholds and wideband tympanometry. Groups were defined as normal hearing (n=92 ears) or hearing loss (n=71 ears) based on ABR thresholds exceeding 15 dB eHL at any test frequency between 500 and 4000 Hz and were further categorized from slight to severe-profound hearing loss. DPOAE level and SNR from 2-8 kHz were evaluated using the Interacoustics Titan, with primary tone stimulus levels set at 65 dB SPL (L1) and 55 dB SPL (L2) and primary tone f2/f1 frequency ratio of 1.22. This study showed that slight or greater hearing loss could be detected accurately, and there was a systematic decrease in DPOAE levels with degree of hearing loss (Figure 1).
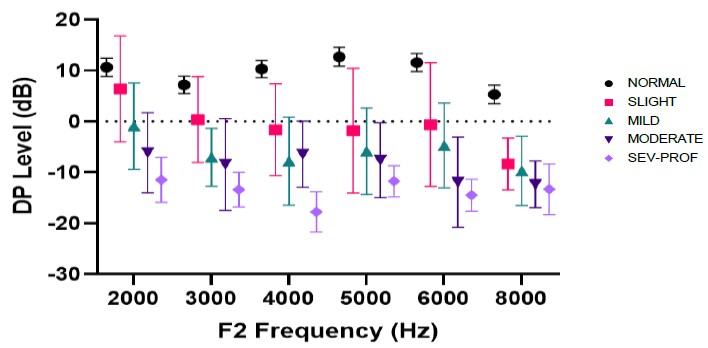
Similar to data in Blankenship et al., DPOAE and SNR levels for optimal detection of hearing loss were greater than adult-based clinical criteria. Optimal cut-point values for detection of hearing loss from this study are shown in Figure 2, compared to the 5th to 95th percentiles of the normal and impaired hearing distributions. It is apparent that for both infants and young children, cut-points that average 0-5 dB across frequencies for DPOAE level provide optimal identification of slight or greater hearing loss. Because newer algorithms improve the SNR, the DPOAE level is important to use as the primary criterion for hearing loss detection. Adequate SNR values (7-10 dB) ensure that the DPOAE levels are interpretable. When both SNR and DPOAE levels are low, and the noise floor is adequate (-5 dB or less), OAEs are interpreted as absent, consistent with hearing impairment.

Finally, it is critical to base the DPOAE level criterion on the appropriate age range. Based on study 3, the same criteria can be employed across the age range from newborns through age 6 years. With improved calibration techniques and probe designs such as employed in the Titan system, these criteria can be applied from 2-8 kHz, as DPOAE levels are relatively constant across that frequency range, and the sensitivity/specificity are also high (.83 to .94 Area Under the Curve). As recommended by Blankenship et al., more than 50% of frequencies assessed should meet DPOAE level and SNR to classify the test as normal overall. Using the combined SNR and DPOAE level criterion across frequencies from 2-8 kHz, sensitivity for detection of hearing loss greater than 15 dB eHL was 94%, and specificity for normal classification was 85%. This approach replicates and validates the results of Blankenship et al. in a larger group of children with a more sensitive criterion for hearing impairment across a larger age range. Use of the SNR criteria of 7-10 dB and the levels above the black line for combined stopping criteria (Table 1) provide the most sensitive and specific detection of slight or greater hearing loss in infants and children up to age 6 years.

Table 1. DPOAE level normative ranges for infants and children (0 months to 6 years, n=92 ears) with normal hearing. Values expressed in dB SPL, rounded to nearest dB. Cut-point values for DP level are the highest area under the receiver curve (AUC) for detection of hearing levels >15 dB eHL.
Frequently Asked Questions
1. What age range is the pediatric DPOAE protocol appropriate for?
The pediatric DPOAE protocol is intended for diagnostic purposes (not for screening) and has been assessed in a diverse clinical population for 2 weeks through age 6 years.
2. Why can’t DPOAEs be used to estimate hearing thresholds?
DPOAEs are related to the degree of hearing loss, but not precisely enough to predict hearing level. OAE level is reduced with increases in pure tone threshold up to a mild degree of hearing loss. Beyond about 40 dB HL, OAEs are often absent because they are most sensitive to mild hearing loss.
3. Why do you use both level and SNR to evaluate DPOAE responses?
The SNR tells you that the OAE is present and can be interpreted. In other words, the OAE is above the noise floor. The level of the DP at each frequency tells you whether the OAE is in the normal hearing or impaired range. The SNR is highly dependent on the person’s internal noise (breathing, blood flow) and noise in the test room. So, it is best used as a measure of test validity. If the SNR is low, retesting after reducing noise and adjusting probe fit may improve the SNR so that the OAE level can be interpreted. When OAEs are absent, they will have low SNRs and low levels. If the noise floor is also low (below -5 dB), then the OAE can be interpreted as absent.
4. What is a cut-off or cut-point?
This is the DP level at a given test frequency that provides the best separation between normal and impaired hearing. In this protocol, we selected SNR and DP level criteria that balanced sensitivity and specificity to detect hearing levels >15 dB eHL.
5. Why are the cut-points different from the lower range of normal (5th percentile)?
The lower boundary of the normal range (5th percentile) gives information about the impaired range, but there is overlap between the normal and impaired groups (gray range). To balance the best sensitivity (detection of hearing impairment) and the best specificity (lowest false-positive rate), we must consider both the normal and the impaired groups. The ideal cut-points improve test sensitivity, so that slight-mild cases of hearing loss are not missed.
6. Why is 1 kHz not included?
Sensitivity and specificity below 2 kHz are lower due to higher noise levels, and test durations are longer, which is not ideal when testing infants and children. However, the protocol using frequencies at 2 kHz and above was able to detect hearing loss at 500 and 1000 Hz as classified by ABR thresholds, because it is rare to have hearing loss only at low frequencies. Even in low frequency hearing loss, OAEs were usually affected at higher frequencies.
7. Is it better to use multiple DP levels, rather than a single test level (e.g., 65/55 dB)?
The use of multiple test levels is known as input-output functions, and can improve prediction of degree of hearing loss, but the test takes longer to complete and requires the child remain quiet for longer periods. Also, because OAEs are frequently absent for hearing levels greater than 40 dB, input-output functions are limited in their predictive power for moderate and greater hearing loss. Research is ongoing in this area and may prove useful in future protocols.
References
1) Abdala, C., Oba, S. I., Ramanathan, R. (2008). Changes in the DP-gram during the preterm and early postnatal period. Ear Hear, 29, 512–523.
2) Blankenship, C. M., L.L. Hunter, D. H. Keefe, M. P. Feeney, D. K. Brown, A. McCune, D. F. Fitzpatrick, and L. Lin. Optimizing clinical interpretation of distortion product otoacoustic emissions in infants. Ear Hear. (2018) Nov/Dec;39(6):1075-1090. PMID: 29517520
3) Dorn, P. A., Piskorski, P., Gorga, M. P., et al. (1999). Predicting audiometric status from distortion product otoacoustic emissions using multivariate analyses. Ear Hear, 20, 149–163.
4) Gorga, M. P., Neely, S. T., Ohlrich, B., et al. (1997). From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear, 18, 440–455.
5) Gorga, M. P., Neely, S. T., Dorn, P. A. (1999). Distortion product otoacoustic emission test performance for a priori criteria and for multifrequency audiometric standards. Ear Hear, 20, 345–362.
6) Gorga, M. P., Dierking, D. M., Johnson, T. A., et al. (2005). A validation and potential clinical application of multivariate analyses of distortion product otoacoustic emission data. Ear Hear, 26, 593–607.
7) Hunter, L.L., C. M. Blankenship, D. H. Keefe, M. P. Feeney, D. K. Brown, A. McCune, D. F. Fitzpatrick, and L. Lin. Longitudinal development of distortion product otoacoustic emissions in infants with normal hearing. Ear Hear. (2018) Sep/Oct;39(5):863-873. PMID: 29369290
8) Hunter, L.L., Sininger, Y.S. Detection of Slight-Mild Hearing Levels with DPOAE and Wideband Tympanometry. Virtual Annual Meeting of the American Auditory Society, March 2021.
9) Sininger YS, Hunter LL, Hayes D, Roush PA, Uhler KM. Evaluation of Speed and Accuracy of Next-Generation Auditory Steady State Response and Auditory Brainstem Response Audiometry in Children with Normal Hearing and Hearing Loss. Ear Hear. 2018 39(6):1207-1223. PMCID: PMC7664445.
Acknowledgements
Funding for studies 1-2 is gratefully acknowledged by the National Institute of Deafness and other Communication Disorders of the National Institutes of Health under Award Number R01 DC010202 and an American Recovery and Reinvestment Act of 2009 supplement (DC010202-01S1) (MPIs Keefe, Feeney and Hunter). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. Funding for study 3 was provided by a research grant from the Oticon Foundation of Denmark (PI Sininger).
Presenter
Get priority access to training
Sign up to the Interacoustics Academy newsletter to be the first to hear about our latest updates and get priority access to our online events.
By signing up, I accept to receive newsletter e-mails from Interacoustics. I can withdraw my consent at any time by using the ‘unsubscribe’-function included in each e-mail.
Click here and read our privacy notice, if you want to know more about how we treat and protect your personal data.
